
Chemistry, 03.09.2019 04:30 michaelbromley9759
The whiye pigment tio2 is prepared by the reaction of titanium tetrachloride, ticl4, with water vapor in the gas phase:
ticl4 + 2h2o2 ? tio + 4hcl
how much heat is evolved in the production of exactly 1 mole of tio2 under standard state conditions?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 15:00
An isotope undergoes radioactive decay by emitting radiation that has no mass. what other characteristic does the radiation have?
Answers: 3

Chemistry, 23.06.2019 16:30
All chemical reactions use reactants in a specific proportion or stoichiometry to form products. the reactant regulates the amount of products produced. a) excess b) limiting c) proportional d) stoichiometric
Answers: 1
You know the right answer?
The whiye pigment tio2 is prepared by the reaction of titanium tetrachloride, ticl4, with water vapo...
Questions



Health, 30.09.2020 02:01

Mathematics, 30.09.2020 02:01






Geography, 30.09.2020 02:01

Health, 30.09.2020 02:01





French, 30.09.2020 02:01




Mathematics, 30.09.2020 02:01

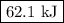
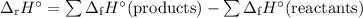
![\begin{array}{rcl}\Delta_{\text{r}}H^{\circ} & = & [-939.7 + 4(-92.307)] - [-763.2 + 2(-241.828)\\& = & [-939.7 - 369.228] - [-763.2 - 483.656]\\& = & -1308.928 + 1246.856\\& = & \mathbf{-62.1}\\\end{array}\\\text{The amount of heat evolved is } \boxed{\textbf{62.1 kJ}}](/tpl/images/0221/6635/abc3a.png)


