
Which of the following states is true? (a) q does not change with temperature. (b) q is the same as keq when a reaction is at equilibrium. (c) key does not change with temperature, whereas q is temperature dependent. (d) k does not depend on the concentration or partial pressures of reaction components. (e) q does not depend on the concentrations or partial pressures of reaction components.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
Which of the following states is true? (a) q does not change with temperature. (b) q is the same as...
Questions


English, 18.02.2021 21:30

Mathematics, 18.02.2021 21:30



Mathematics, 18.02.2021 21:30





English, 18.02.2021 21:30


Arts, 18.02.2021 21:30



Biology, 18.02.2021 21:30


Computers and Technology, 18.02.2021 21:30


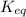 is the equilibrium constant and it is also equal to the concentration of products divided by the concentration of reactants. Also,
is the equilibrium constant and it is also equal to the concentration of products divided by the concentration of reactants. Also, 

