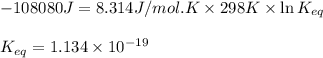Chemistry, 03.09.2019 05:10 kjmccarty02
Determine the direction in which the following reaction is spontaneous at 25oc: mg2+(aq) + k(s) < > mg(s) + k+(aq) determine the equilibrium constant k and go using the cell potential for this reaction and which will be the anode and cathode?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Where are each of the three particles located within the atom?
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
You know the right answer?
Determine the direction in which the following reaction is spontaneous at 25oc: mg2+(aq) + k(s) <...
Questions




Mathematics, 10.11.2020 04:40

Mathematics, 10.11.2020 04:40



Mathematics, 10.11.2020 04:40

Mathematics, 10.11.2020 04:40

Biology, 10.11.2020 04:40




Mathematics, 10.11.2020 04:40

Business, 10.11.2020 04:40



Mathematics, 10.11.2020 04:40


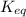 and
and 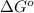 of the reaction is
of the reaction is 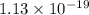 and -108080 J respectively.
and -108080 J respectively. 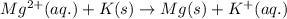
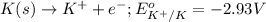
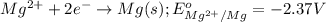
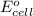 of the reaction, we use the equation:
of the reaction, we use the equation: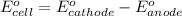
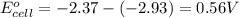
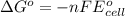
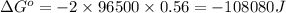
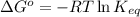
![25^oC=[273+25]=298K](/tpl/images/0221/6855/6a9f9.png)