
Propane c3h8 is a hydrocarbon tht is commonly used as a fuel
(a)write a balanced equation for the complete combustion used propane gas.
(b) calculate the volume of air at 25c and 1.00 atmosphere that is needed to completely combust 25.0 grams of propna. assume that air is 21.0 percnt o2 by volume. (hint: we will see how to do this calcullation in a later chapter on gases -- for now use the infomation that 1.00l of air at 25c and 1.00atm contains 0.275 g of o2 pe liter.)
(c) the heat of combustion of propane is -2,219.2 kj/mol. calculate the heat of formation, hof of propane given that hof of h2o = -285.8 kj/mol and hof of co2 = -393.5 kj/mol
(d) assuming that all of the het releasd in burning 25.0 grams of propane is transferred to 4.00 kilograms of water calculate the increase in temperature of water .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 23.06.2019 04:31
How many grams of iron can be made from 16.5 grams of fe2o3
Answers: 1

Chemistry, 23.06.2019 09:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2

Chemistry, 23.06.2019 10:50
Gene expression control that occurs during the generation of rna is a. controlled at transcription b. control before transcription c. controlled after transcription d. controlled after translation
Answers: 3
You know the right answer?
Propane c3h8 is a hydrocarbon tht is commonly used as a fuel
(a)write a balanced equation for...
(a)write a balanced equation for...
Questions




Mathematics, 06.06.2020 17:58











Computers and Technology, 06.06.2020 17:59





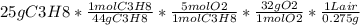
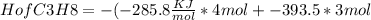
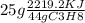 = 1260.9KJ
= 1260.9KJ

