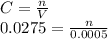Chemistry, 03.09.2019 21:30 champions2k19
A25.0-ml sample containing cu2+ gave an instrument signal of 25.2 units (corrected for a blank). when exactly 0.500 ml of 0.0275 m cu(no3)2 was added to the solution, the signal increased to 45.1 units. calculate the molar concentration of cu2+ assuming that the signal was directly proportional to the analyte concentration. skoog, douglas a.. principles of instrumental analysis (p. 20). brooks cole. kindle edition.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
A25.0-ml sample containing cu2+ gave an instrument signal of 25.2 units (corrected for a blank). whe...
Questions


Mathematics, 09.02.2021 17:00


Computers and Technology, 09.02.2021 17:00

Mathematics, 09.02.2021 17:00

Mathematics, 09.02.2021 17:00


Mathematics, 09.02.2021 17:00




Mathematics, 09.02.2021 17:10


English, 09.02.2021 17:10

English, 09.02.2021 17:10

Mathematics, 09.02.2021 17:10



Mathematics, 09.02.2021 17:10