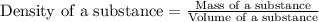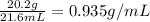Chemistry, 03.09.2019 23:30 iibabycarrotsii
To save time you can approximate the initial volume of water to ±1 ml and the initial mass of the solid to ±1 g. for example, if you are asked to add 23 ml of water, add between 22 ml and 24 ml. which metals in each of the following sets will have equal density? *20.2 g gold placed in 21.6 ml of water and 12.0 g copper placed in 21.6 ml of water. *20.2 g silver placed in 21.6 ml of water and 12.0 g silver placed in 21.6 ml of water. *15.2 g copper placed in 21.6 ml of water and 50.0 g copper placed in 23.4 ml of water. *15.4 g gold placed in 20.0 ml of water and 15.7 g silver placed in 20.0 ml of water. *20.2 g silver placed in 21.6 ml of water and 20.2 g copper placed in 21.6 ml of water. *11.2 g gold placed in 21.6 ml of water and 14.9 g gold placed in 23.4 ml of water.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
To save time you can approximate the initial volume of water to ±1 ml and the initial mass of the so...
Questions



Spanish, 18.02.2020 22:59







Biology, 18.02.2020 22:59

Mathematics, 18.02.2020 22:59


Computers and Technology, 18.02.2020 22:59

Biology, 18.02.2020 22:59






History, 18.02.2020 22:59