Chemistry, 04.09.2019 00:10 ashley5196
Sulfuric acid (h2so4) is prepared commercially from elemental sulfur using the contact process. in a typical sequence of reactions, the sulfur is first burned: s + o2 → so2 , then it is converted to so3 using a catalyst: 2 so2 + o2 → 2 so3 . the resulting so3 is reacted with water to produce the desired product: so3 + h2o → h2so4 . how much sulfuric acid could be prepared from 83 moles of sulfur? answer in units of g.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
Sulfuric acid (h2so4) is prepared commercially from elemental sulfur using the contact process. in a...
Questions











Mathematics, 26.02.2020 20:14




Biology, 26.02.2020 20:14

Social Studies, 26.02.2020 20:15




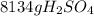
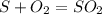
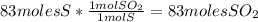
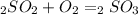
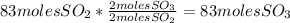
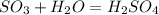
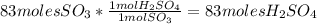
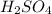 :
: