
Chemistry, 04.09.2019 00:30 wyattjefferds05
Trevor dissolves sodium hydroxide pellets in a beaker of water at room temperature, and notes that the beaker becomes warm. which correctly designates the signs of δh, δs, and δg for this process?
a. δh > 0, δs > 0, and δg < 0
b. δh < 0, δs > 0, and δg < 0
c. δh > 0, δs > 0, and δg > 0
d. δh < 0, δs < 0, and δg > 0

Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:04
What is the smallest component or the most basic building block of any element ? a. an atom, b.a compound c.gas d.element
Answers: 1

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2
You know the right answer?
Trevor dissolves sodium hydroxide pellets in a beaker of water at room temperature, and notes that t...
Questions

Mathematics, 16.08.2021 14:00


English, 16.08.2021 14:00


History, 16.08.2021 14:00

Geography, 16.08.2021 14:00

History, 16.08.2021 14:00

Physics, 16.08.2021 14:00

World Languages, 16.08.2021 14:00


Geography, 16.08.2021 14:00

Social Studies, 16.08.2021 14:00

Physics, 16.08.2021 14:00

English, 16.08.2021 14:00

Biology, 16.08.2021 14:00

Business, 16.08.2021 14:00

Mathematics, 16.08.2021 14:00


Physics, 16.08.2021 14:00

Mathematics, 16.08.2021 14:00

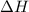 for Exothermic reaction is negative and
for Exothermic reaction is negative and 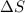 is positive when randomness increases and
is positive when randomness increases and 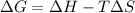
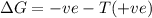
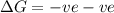
 will be negative.
will be negative.

