
Chemistry, 04.09.2019 02:30 peytonbrien2002
Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. when 4.51 g of magnesium ribbon burns with 6.92 g of oxygen, a bright, white light and a white, powdery product are formed. enter the balanced chemical equation for this reaction. be sure to include all physical states.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 23.06.2019 08:30
Which can be observed only in a microscopic view? a) structure of a muscle cell b) shape of a soybean plant c) foam insulation d) x-ray of a knee joint
Answers: 2

Chemistry, 23.06.2019 11:00
What are the other two pieces of glassware you used in this experiment that you could obtain hundredths digit accuracy?
Answers: 2
You know the right answer?
Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. when 4...
Questions








English, 16.04.2020 22:38



Chemistry, 16.04.2020 22:38









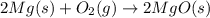
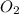 is present in gaseous state, and MgO is present in solid state.
is present in gaseous state, and MgO is present in solid state.


