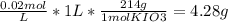Chemistry, 04.09.2019 03:20 JosefineRubino2204
You wish to prepare 1 l of a 0.02 m potassium iodate solution. you require that the final concentration be within 1% of 0.02 m and that the concentration must be known accurately to the fourth decimal place. how would you prepare this solution? specify the glassware you would use, the accuracy needed for the balance, and the ranges of acceptable masses of kio3 that could be used.(a) to make this solution (ideally) you would need grams of potassium iodide dissolved in enough water to make up 1 l of solution. fill in the blank(b)what is the least accurate balance that could be used to make this solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1


Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
You wish to prepare 1 l of a 0.02 m potassium iodate solution. you require that the final concentrat...
Questions

English, 11.03.2021 18:50


Mathematics, 11.03.2021 18:50


Mathematics, 11.03.2021 18:50

Mathematics, 11.03.2021 18:50

English, 11.03.2021 18:50


Arts, 11.03.2021 18:50




Mathematics, 11.03.2021 18:50


Mathematics, 11.03.2021 18:50

Social Studies, 11.03.2021 18:50

Mathematics, 11.03.2021 18:50


Mathematics, 11.03.2021 18:50