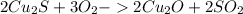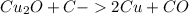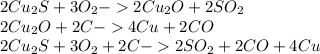Chemistry, 04.09.2019 04:10 trevorhenyan51
There are two steps in the extraction of copper metal from chalcocite, a copper ore. in the first step, copper(i) sulfide and oxygen react to form copper(i) oxide and sulfur dioxide. in the second step, copper(i) oxide and carbon react to form copper and carbon monoxide. write the net chemical equation for the production of copper from copper(i) sulfide, oxygen and carbon. be sure your equation is balanced.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
There are two steps in the extraction of copper metal from chalcocite, a copper ore. in the first st...
Questions





Mathematics, 03.08.2019 09:00

Chemistry, 03.08.2019 09:00

History, 03.08.2019 09:00


History, 03.08.2019 09:00

Mathematics, 03.08.2019 09:00

Spanish, 03.08.2019 09:00

Chemistry, 03.08.2019 09:00


Business, 03.08.2019 09:00


Mathematics, 03.08.2019 09:00

Mathematics, 03.08.2019 09:00

Mathematics, 03.08.2019 09:00

History, 03.08.2019 09:00

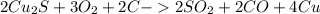
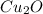 , that later reacts with C, to poduce the copper metal.
, that later reacts with C, to poduce the copper metal.