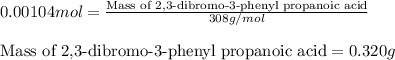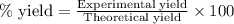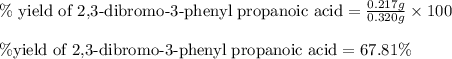Suppose a student started with 155.0 mg of trans-cinnamic acid, 458 mg of pyridinium tribromide, and 2.35 ml of glacial acetic acid. after the reaction and workup, the student ended up with 0.2170 g of brominated product. calculate the student\'s theoretical and percent yields.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
Suppose a student started with 155.0 mg of trans-cinnamic acid, 458 mg of pyridinium tribromide, and...
Questions

Arts, 02.10.2020 09:01

Computers and Technology, 02.10.2020 09:01




Mathematics, 02.10.2020 09:01



Physics, 02.10.2020 09:01

Biology, 02.10.2020 09:01

English, 02.10.2020 09:01


Mathematics, 02.10.2020 09:01


Mathematics, 02.10.2020 09:01

Mathematics, 02.10.2020 09:01


Mathematics, 02.10.2020 09:01

Geography, 02.10.2020 09:01

Mathematics, 02.10.2020 09:01

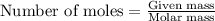 .....(1)
.....(1)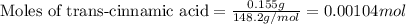
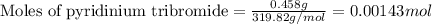
![\text{trans-cinnamic acid}+\text{pyridinium tribromide}\xrightarrow[]{CH_3COOH}\text{2,3-dibromo-3-phenyl propanoic acid}](/tpl/images/0222/4310/95be0.png)
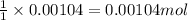 of pyridinium tribromide
of pyridinium tribromide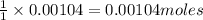 of 2,3-dibromo-3-phenyl propanoic acid
of 2,3-dibromo-3-phenyl propanoic acid