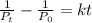Hi(g) decomposes to molecular hydrogen and molecular iodine by a
second-order reaction. if the initial pressure of hl is 0.691 atm and
the rate constant for the reaction is 0.100 atm-1.min-1, how much
time (min) will pass before the hi pressure drops to 0.187 atm?
enter your answer to 1 decimal place.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1


Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1
You know the right answer?
Hi(g) decomposes to molecular hydrogen and molecular iodine by a
second-order reaction. if the...
second-order reaction. if the...
Questions

Arts, 07.06.2021 16:20

Mathematics, 07.06.2021 16:20



Mathematics, 07.06.2021 16:30


Mathematics, 07.06.2021 16:30

Mathematics, 07.06.2021 16:30





English, 07.06.2021 16:30

Computers and Technology, 07.06.2021 16:30


Computers and Technology, 07.06.2021 16:30

English, 07.06.2021 16:30

Biology, 07.06.2021 16:30


History, 07.06.2021 16:30

![\int\limits^{[HI_1]}_{[HI_0]} {\,\frac{d[HI]}{[HI]^2}=-\int\limits^t_0 {t} \, dt](/tpl/images/0222/4956/40b7f.png)
![\frac{1}{[HI_t]}-\frac{1}{[HI_0]}=kt](/tpl/images/0222/4956/6b6cb.png)