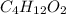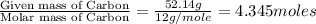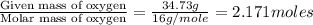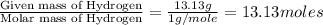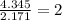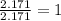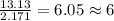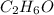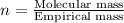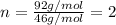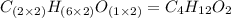Chemistry, 05.09.2019 01:10 fernandaretanaoxwln0
Determine the empirical formula for a compound that contains c, h and o. it contains 52.14% c and 34.73% o by mass. what is the molecular formula if the molar mass is 92 g/mol?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1
You know the right answer?
Determine the empirical formula for a compound that contains c, h and o. it contains 52.14% c and 34...
Questions


Mathematics, 18.10.2020 02:01


Mathematics, 18.10.2020 02:01

Mathematics, 18.10.2020 02:01

Arts, 18.10.2020 02:01

Mathematics, 18.10.2020 02:01

Mathematics, 18.10.2020 02:01

English, 18.10.2020 02:01


Mathematics, 18.10.2020 02:01

English, 18.10.2020 02:01




History, 18.10.2020 02:01

History, 18.10.2020 02:01

Mathematics, 18.10.2020 02:01


Mathematics, 18.10.2020 02:01