Using the standard enthalpies of formation, what is the standard enthalpy of reaction?
co(g)...

Chemistry, 05.09.2019 02:30 kaylienguyen
Using the standard enthalpies of formation, what is the standard enthalpy of reaction?
co(g)+h2o(g)⟶co2(g)+h2(g)
the formation values are as follows:
δhf (co(g)) = −110.525kj/mol
δhf (co2(g)) = −393.509kj/mol
δhf (h2o(g)) = −241.818kj/mol
δhf (h2(g)) = 0

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 10:30
Rocks, as they are compressed, begin forming mountains above the earth's surface when two continental plates converge. the continental crust increases in depth as the mountains grow above. the himalayan mountains formed at a convergent plate boundary in this manner. the rocks are smashed together causing them to due to the intense heat and pressure from the colliding plates and eventually forming rock. a) melt; igneous b) layer; sedimentary c) recrystallize; metamorphic d) melt into the earth's interior; metamorphic
Answers: 1

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
Questions

Biology, 04.02.2020 11:48


Mathematics, 04.02.2020 11:48

Mathematics, 04.02.2020 11:48



History, 04.02.2020 11:48

Health, 04.02.2020 11:48

Health, 04.02.2020 11:48


Mathematics, 04.02.2020 11:48



Geography, 04.02.2020 11:48



Business, 04.02.2020 11:48


Mathematics, 04.02.2020 11:48

![\sum [n_{i}\times \Delta H_{f}(product)_{i}]-\sum [n_{j}\times \Delta H_{f}(reactant)_{j}]](/tpl/images/0223/2160/eef77.png)
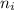 and
and 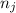 are number of moles of i-th product and j-th reactant in balanced reaction respectively.
are number of moles of i-th product and j-th reactant in balanced reaction respectively.![[(1\times \Delta H_{f}(CO_{2})_{g})]+[(1\times \Delta H_{f}(H_{2})_{g})]-[(1\times \Delta H_{f}(CO)_{g})]-[(1\times \Delta H_{f}(H_{2}O)_{g})]](/tpl/images/0223/2160/f000a.png)
![[(1\times -393.509]+[(1\times 0]-[(1\times -110.525]-[(1\times -241.818]](/tpl/images/0223/2160/cf695.png) kJ = -41.166 kJ
kJ = -41.166 kJ

