
Chemistry, 05.09.2019 03:30 greyxxamber
The equilibrium constant for a reaction is 0.48 at 25 °c. what is the value of δg° (kj/mol) at this temperature?
(a) 1.8
(b) -4.2
(c) 1.5 x 10^2
(d) 4.2
(e) more information is needed.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1


Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
The equilibrium constant for a reaction is 0.48 at 25 °c. what is the value of δg° (kj/mol) at this...
Questions


Biology, 12.03.2021 19:50

Mathematics, 12.03.2021 19:50

Mathematics, 12.03.2021 19:50

Mathematics, 12.03.2021 19:50

History, 12.03.2021 19:50

Biology, 12.03.2021 19:50

Mathematics, 12.03.2021 19:50





Mathematics, 12.03.2021 19:50

Mathematics, 12.03.2021 19:50

Social Studies, 12.03.2021 19:50


Mathematics, 12.03.2021 19:50


Biology, 12.03.2021 19:50

English, 12.03.2021 19:50

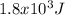
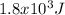 or 1.8 kJ
or 1.8 kJ

