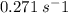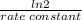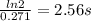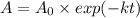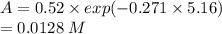Chemistry, 05.09.2019 04:10 mollykay2001p3qo0j
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1.
(a) what is the half-life for this reaction?
(b) if you start with 0.052 m i2 at this temperature, how much will remain after 5.16 s assuming that the iodine atoms do not recombine to form i2?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
How many atoms are in 1.4 mil of phosphorus trifluoride (pf3)
Answers: 3

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1
You know the right answer?
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of...
Questions

Mathematics, 09.12.2020 22:50




Mathematics, 09.12.2020 22:50




Spanish, 09.12.2020 22:50





Mathematics, 09.12.2020 22:50

Chemistry, 09.12.2020 22:50



Advanced Placement (AP), 09.12.2020 22:50

Mathematics, 09.12.2020 22:50

English, 09.12.2020 22:50