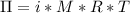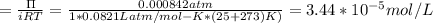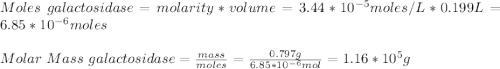Chemistry, 05.09.2019 16:10 elizabethseoane1829
A0.797 g sample of β‑galactosidase is dissolved in water to make 0.199 l of solution, and the osmotic pressure of the solution at 25 ∘c is found to be 0.853 mbar. calculate the molecular mass of β‑galactosidase.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2
You know the right answer?
A0.797 g sample of β‑galactosidase is dissolved in water to make 0.199 l of solution, and the osmoti...
Questions


Mathematics, 01.06.2020 00:57

Mathematics, 01.06.2020 00:57

Mathematics, 01.06.2020 00:57



Mathematics, 01.06.2020 00:57



History, 01.06.2020 00:57




Mathematics, 01.06.2020 00:57

Mathematics, 01.06.2020 00:57


Mathematics, 01.06.2020 00:57