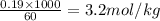Chemistry, 05.09.2019 16:20 hardwick744
Asolution is prepared by dissolving 31.9 g cesium chloride in 60.0 g water. the volume of the solution is 63.3 ml. calculate the molality of the cscl solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
You know the right answer?
Asolution is prepared by dissolving 31.9 g cesium chloride in 60.0 g water. the volume of the soluti...
Questions

Mathematics, 27.11.2020 01:10

Business, 27.11.2020 01:10



Mathematics, 27.11.2020 01:10

English, 27.11.2020 01:10

Mathematics, 27.11.2020 01:10

Mathematics, 27.11.2020 01:10

Mathematics, 27.11.2020 01:10


History, 27.11.2020 01:10


Mathematics, 27.11.2020 01:10






Mathematics, 27.11.2020 01:10

Mathematics, 27.11.2020 01:10

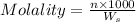
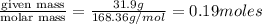
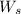 = weight of solvent (water) in g = 60.0 g
= weight of solvent (water) in g = 60.0 g