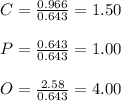Measurements show that unknown compound has the following composition: element mass 38.7 % calcium, 19.9 % phosphorus and 41.2 % oxygen. write the empirical chemical formula of this compound?
(a) ca2po4
(b) ca3po6
(c) ca4p2o4
(d) ca3p2o8 (or ca3(po4)2)
(e) capo4

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
Measurements show that unknown compound has the following composition: element mass 38.7 % calcium,...
Questions

Mathematics, 03.08.2019 04:30


Arts, 03.08.2019 04:30










Social Studies, 03.08.2019 04:30




Health, 03.08.2019 04:30


Arts, 03.08.2019 04:30