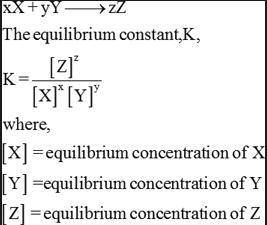
An aqueous solution of acetic acid is found to have the following equilibrium concentrations at 25 c:
[ch3cooh] = 1.65 x 10^-2 m; [h+] = 5.44 x 10^-4 m; and [ch3coo-] = 5.44 x 10^-4 m. calculate the equilibrium constant kc for the ionization of acetic acid at 25 c. the reaction is
ch3cooh -> h+ + ch3coo-

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1
You know the right answer?
An aqueous solution of acetic acid is found to have the following equilibrium concentrations at 25 c...
Questions


Physics, 08.10.2021 14:00

Arts, 08.10.2021 14:00


Mathematics, 08.10.2021 14:00



Biology, 08.10.2021 14:00

Mathematics, 08.10.2021 14:00

Biology, 08.10.2021 14:00


Chemistry, 08.10.2021 14:00


Health, 08.10.2021 14:00

Social Studies, 08.10.2021 14:00

Mathematics, 08.10.2021 14:00

Advanced Placement (AP), 08.10.2021 14:00

Mathematics, 08.10.2021 14:00


![Kc=\frac{[H+] [CH3COO-]}{[CH3COOH]}](/tpl/images/0223/5183/bf641.png)