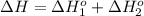Chemistry, 05.09.2019 19:30 calindaperez
Calculate δh (in kj) for the process hg2br2(s) → 2 hg(l) + br2(l) from the following information.
hg(l) + br2(l) → hgbr2(s) δh⁰298 = −170.7 kj
hg(l) + hgbr2(s) → hg2br2(s) δh⁰298 = −36.2 kj

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3
You know the right answer?
Calculate δh (in kj) for the process hg2br2(s) → 2 hg(l) + br2(l) from the following information.
Questions


Chemistry, 01.02.2021 21:10


Mathematics, 01.02.2021 21:10


Health, 01.02.2021 21:10




Mathematics, 01.02.2021 21:10

Social Studies, 01.02.2021 21:10



Law, 01.02.2021 21:10




Mathematics, 01.02.2021 21:10

History, 01.02.2021 21:10