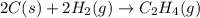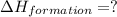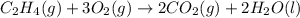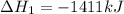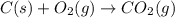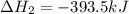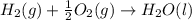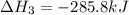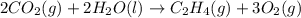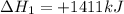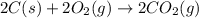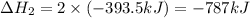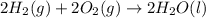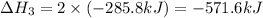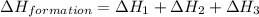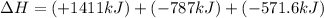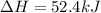Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1
You know the right answer?
Find the standard enthalpy of formation of ethylene, c2h4(g), given the following data: c2h4(g) + 3...
Questions

Physics, 15.01.2021 16:40

Mathematics, 15.01.2021 16:40




Mathematics, 15.01.2021 16:40


Mathematics, 15.01.2021 16:40

English, 15.01.2021 16:40

History, 15.01.2021 16:40





Mathematics, 15.01.2021 16:40

Mathematics, 15.01.2021 16:40


Mathematics, 15.01.2021 16:40


Social Studies, 15.01.2021 16:40

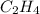 will be,
will be,