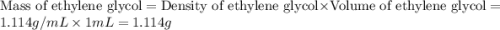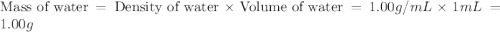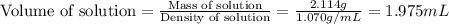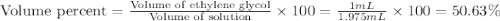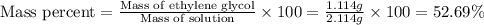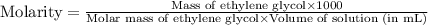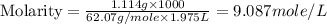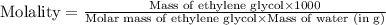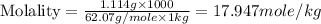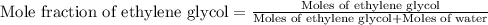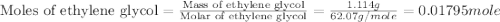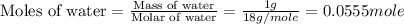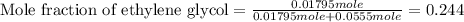An automobile antifreeze mixture is made by mixing equal volumes of ethylene glycol (d = 1.114 g/ml; m = 62.07 g/mol) and water (d = 1.00 g/ml) at 20°c. the density of the mixture is 1.070 g/ml. express the concentration of ethylene glycol as
a- volume percent
b- mass percent
c- molarity
d- molality
e- mole fraction

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 19:00
Imagine that a new planet is discovered with two moons of equal mass: moon a and moon b. the mass of the new planet is greater than the combined mass of its moons. moon a is farther away from the new planet than moon b. what is the planet's gravitational pull on moon a compared to the planet's gravitational pull on moon b? the planet's gravity repels moon a with a greater force than it repels moon b, which is why moon a is farther away. the gravitational pull on moon b is greater than on moon a because moon b is closer to the new planet than moon a. the gravitational pull on moon b is greater than on moon a because moon b is farther away from the new planet than moon a. the gravitational pull on moon a is the same as the gravitational pull on moon b because distance does not affect the planet's gravity.
Answers: 1
You know the right answer?
An automobile antifreeze mixture is made by mixing equal volumes of ethylene glycol (d = 1.114 g/ml;...
Questions

History, 01.07.2020 15:01

Health, 01.07.2020 15:01

History, 01.07.2020 15:01




Arts, 01.07.2020 15:01


English, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01

Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01


English, 01.07.2020 15:01

History, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01

Mathematics, 01.07.2020 15:01