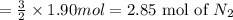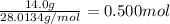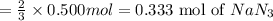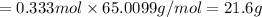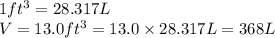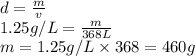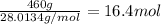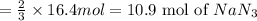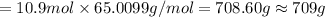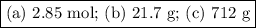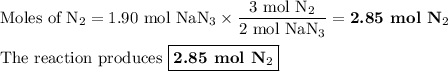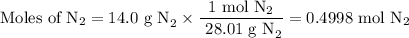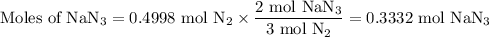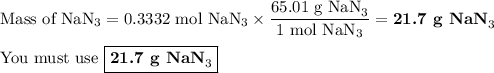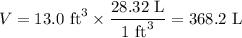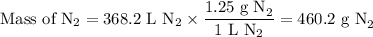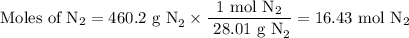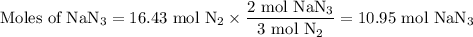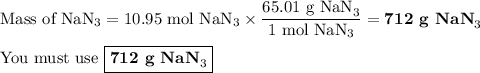Chemistry, 05.09.2019 22:30 emilyturchon
Automotive air bags inflate when sodium azide, nan3, rapidly decomposes to its component elements: 2nan3(s)→2na(s)+3n2(g)
(a) how many moles of n2 are produced by the decomposition of 1.90 mol of nan3?
(b) how many grams of nan3 are required to form 14.0 g of nitrogen gas?
(c) how many grams of nan3 are required to produce 13.0 ft3 of nitrogen gas if the gas has a density of 1.25 g/l?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
Automotive air bags inflate when sodium azide, nan3, rapidly decomposes to its component elements:...
Questions


Biology, 22.05.2021 08:20

Business, 22.05.2021 08:20

Mathematics, 22.05.2021 08:20



Biology, 22.05.2021 08:20

Chemistry, 22.05.2021 08:20

Mathematics, 22.05.2021 08:20

Business, 22.05.2021 08:20

Mathematics, 22.05.2021 08:20




Mathematics, 22.05.2021 08:20

English, 22.05.2021 08:20


Biology, 22.05.2021 08:20

Mathematics, 22.05.2021 08:20

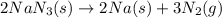
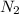 produced by the decomposition of 1.90 mol of
produced by the decomposition of 1.90 mol of 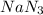 .
.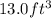 of nitrogen gas if the gas has a density of 1.25 g/L.
of nitrogen gas if the gas has a density of 1.25 g/L.