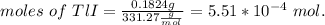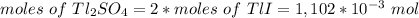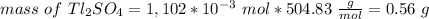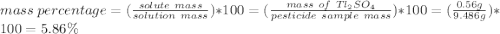Chemistry, 05.09.2019 22:30 Ashley606hernandez
The thallium (present as tl2so4) in a 9.486-g pesticide sample was precipitated as thallium(i) iodide. calculate the mass percent of tl2so4 in the sample if 0.1824 g of tli was recovered.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2


Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
The thallium (present as tl2so4) in a 9.486-g pesticide sample was precipitated as thallium(i) iodid...
Questions

Mathematics, 27.06.2019 03:30










English, 27.06.2019 03:30



History, 27.06.2019 03:30

History, 27.06.2019 03:30

Mathematics, 27.06.2019 03:30

History, 27.06.2019 03:30

Mathematics, 27.06.2019 03:30

Computers and Technology, 27.06.2019 03:30

Mathematics, 27.06.2019 03:30

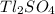 is 5.86%
is 5.86%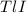 precipitate. We can establish a relation between the mass of
precipitate. We can establish a relation between the mass of