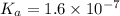Amonoprotic weak acid, ha , dissociates in water according to the reaction ha(aq)↽−−⇀h+(aq)+a−(aq) the equilibrium concentrations of the reactants and products are [ha]=0.250 m , [h+]=2.00×10−4 m , and [a−]=2.00×10−4 m . calculate the value of pka for the acid ha .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
Amonoprotic weak acid, ha , dissociates in water according to the reaction ha(aq)↽−−⇀h+(aq)+a−(aq) t...
Questions

Mathematics, 25.11.2020 01:00



Mathematics, 25.11.2020 01:00






Mathematics, 25.11.2020 01:00

Biology, 25.11.2020 01:00



Social Studies, 25.11.2020 01:00

History, 25.11.2020 01:00

Advanced Placement (AP), 25.11.2020 01:00

Mathematics, 25.11.2020 01:00


Biology, 25.11.2020 01:00

 of HA is 6.80
of HA is 6.80
 ) of HA is represented as-
) of HA is represented as-![K_{a}=\frac{[H^{+}][A^{-}]}{[HA]}](/tpl/images/0223/8979/c814f.png)