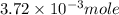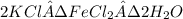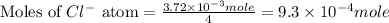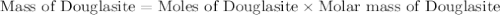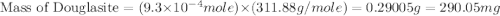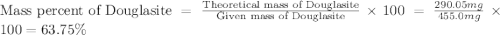Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Douglasite is a mineral with the formula 2kcl # fecl2 # 2h2o. calculate the mass percent of douglasi...
Questions


Chemistry, 01.03.2021 22:50




Social Studies, 01.03.2021 22:50






Mathematics, 01.03.2021 22:50

History, 01.03.2021 22:50







Arts, 01.03.2021 22:50

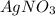 .
.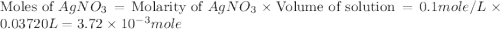
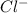 =
=