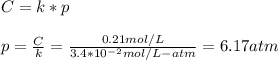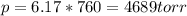Chemistry, 05.09.2019 23:30 avalianagames
Part a an unopened can of soda has an aqueous co2 concentration of 0.21 m at 25.0 °c. what is the partial pressure of the gas in the can in torr? the henry's law constant for co2 at 25 °c is 3.4 × 10−2 mol/l-atm.

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 09:30
If the solubility of a gas in water is 1.22g/2.75 atm, what is it’s solubility (in g/l) at 1.0 atm
Answers: 1

Chemistry, 23.06.2019 09:50
T(s) in2os] (m) 0 185 2.39 546 1.90 725 1.70 the decomposition of n205 can be described by the equation 2.68 given these data for the reaction at 45°c in carbon tetrachloride solution, calculate the average rate of reaction for each successive time interval. ntr s to 185 s 185 s to 546 s 546 s to 725 s number number number reaction rate: m/s m/s m/s
Answers: 1
You know the right answer?
Part a an unopened can of soda has an aqueous co2 concentration of 0.21 m at 25.0 °c. what is the pa...
Questions

Mathematics, 30.08.2019 20:30


Computers and Technology, 30.08.2019 20:30



Biology, 30.08.2019 20:30




Social Studies, 30.08.2019 20:30

Social Studies, 30.08.2019 20:30

Mathematics, 30.08.2019 20:30

Advanced Placement (AP), 30.08.2019 20:30

History, 30.08.2019 20:30

Mathematics, 30.08.2019 20:30




English, 30.08.2019 20:30