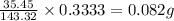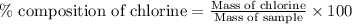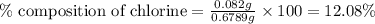The percent chloride in an unknown sample may be determined by gravimetric methods. suppose a 0.6789g sample of an unknown chloride sample was dissolved and agcl is precipitated by adding agno3 solution. the precipitate was filtered, ignited, and found to weigh 0.g. what was the percent chloride in the sample?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1
You know the right answer?
The percent chloride in an unknown sample may be determined by gravimetric methods. suppose a 0.6789...
Questions




Mathematics, 26.07.2021 15:10


Business, 26.07.2021 15:10


English, 26.07.2021 15:10

Computers and Technology, 26.07.2021 15:10


Social Studies, 26.07.2021 15:10


Mathematics, 26.07.2021 15:20

Arts, 26.07.2021 15:20

Mathematics, 26.07.2021 15:20


Mathematics, 26.07.2021 15:20