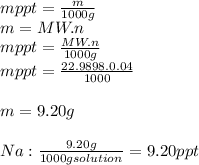
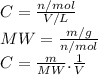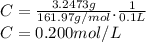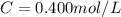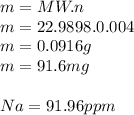3.2473 g of na2cro4 (mw = 161.97 g/mol) is dissolved in 100.0 ml of water. assuming the solution has a density of 1.00 g/ml, what is the concentration of na (mw = 22.9898 g/mol) in the solution in units of (a) molarity (m)? (b) parts per thousand (ppt)? (c) 10.0 ml of the solution is then diluted to a final volume of 1000.0 ml. what is the concentration of na in the diluted solution in units of parts per million (ppm)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
3.2473 g of na2cro4 (mw = 161.97 g/mol) is dissolved in 100.0 ml of water. assuming the solution has...
Questions





Mathematics, 24.01.2021 20:00

Spanish, 24.01.2021 20:00

Mathematics, 24.01.2021 20:00

Mathematics, 24.01.2021 20:00

Mathematics, 24.01.2021 20:00


History, 24.01.2021 20:00

Mathematics, 24.01.2021 20:00

Mathematics, 24.01.2021 20:00

Mathematics, 24.01.2021 20:00



Mathematics, 24.01.2021 20:00

Social Studies, 24.01.2021 20:00

English, 24.01.2021 20:00

World Languages, 24.01.2021 20:00