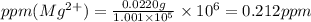Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
Wastewater from a cement factory contains 0.280 g of ca2+ ion and 0.0220 g of mg2+ ion per 100.0 l o...
Questions








Mathematics, 07.04.2020 16:53












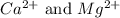 ions are 2.797 ppm and 0.212 ppm respectively.
ions are 2.797 ppm and 0.212 ppm respectively.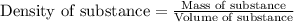
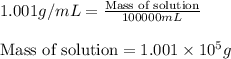
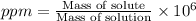
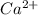 ions = 0.280 g
ions = 0.280 g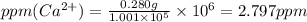
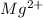 ions = 0.0220 g
ions = 0.0220 g