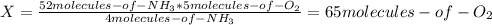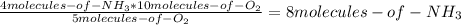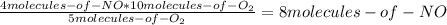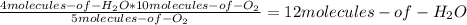Consider the following reaction: 4nh3(g) + 5o2(g) → 4no(g) + 6h2o(g) if a container were to have 10 molecules of o2 and 52 molecules of nh3 initially, how many total molecules (reactants plus products) would be present in the container after this reaction goes to completion?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Which of the following statements concerning the influence of culture on ethnic identity formation is accurate? a. one will reject ethnic identity if cultural stereotypes are encountered. b. if one’s ethnic city is different from the dominant cultural group, then one’s ethnic identity you will become weekend. c. if an the ethnic group is excepted by dominant culture, then ethnic identity formation can be a difficult process. d. similarity to the dominant culture can determine how easy it is for one to except ethnic differences.
Answers: 2


Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

You know the right answer?
Consider the following reaction: 4nh3(g) + 5o2(g) → 4no(g) + 6h2o(g) if a container were to have 10...
Questions





Mathematics, 03.07.2021 19:30

Mathematics, 03.07.2021 19:30

Biology, 03.07.2021 19:30

World Languages, 03.07.2021 19:30




Mathematics, 03.07.2021 19:30

Biology, 03.07.2021 19:30