
Chemistry, 06.09.2019 17:20 kookycookiefanx
The chemical equation for respiration of a molecule of glucose is: c6h12o6 + 6o2 -> 6co2 + 6 h2o. this is the same reaction as when a marshmallow catches fire and burns. in this oxidation-reduction reaction, which molecule(s) is(are) reduced?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
The chemical equation for respiration of a molecule of glucose is: c6h12o6 + 6o2 -> 6co2 + 6 h2...
Questions


Chemistry, 25.09.2019 07:30

History, 25.09.2019 07:30

Biology, 25.09.2019 07:30

Physics, 25.09.2019 07:30



English, 25.09.2019 07:30

Mathematics, 25.09.2019 07:30

Biology, 25.09.2019 07:30

Mathematics, 25.09.2019 07:30

English, 25.09.2019 07:30


Chemistry, 25.09.2019 07:30




Biology, 25.09.2019 07:30

Biology, 25.09.2019 07:30

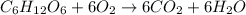
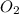 gets attached to two hydrogen atoms.
gets attached to two hydrogen atoms.

