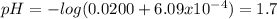A) a solution consists of 0.25 m hydrofluoric acid (hf) and 0.28 m sodium fluoride (naf). the k of hydrofluoric acid acid is 6.8 x 10"", calculate the ph of the solution. b) to one liter of the solution from part (a) is added 0.0200 moles of perchloric acid (hcio4). there is no change in the total volume. calculate the ph after the addition of the perchloric acid.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2


Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
A) a solution consists of 0.25 m hydrofluoric acid (hf) and 0.28 m sodium fluoride (naf). the k of h...
Questions



Mathematics, 07.04.2020 21:21


Mathematics, 07.04.2020 21:21


History, 07.04.2020 21:21

English, 07.04.2020 21:21


Social Studies, 07.04.2020 21:21










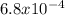 so you can write the equation:
so you can write the equation: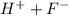
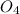 is a strong acid and completely dissociates.
is a strong acid and completely dissociates.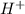 and x moles of
and x moles of 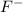 and you can write K like this:
and you can write K like this: ![K=\frac{[H^{+}][F^{-}] }{[HF]}](/tpl/images/0224/4748/809f2.png) . note that
. note that 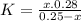 and solving for x, x=6.09x
and solving for x, x=6.09x 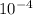 M
M![[H^{+} ]](/tpl/images/0224/4748/cd271.png) and you are abble to find pH with
and you are abble to find pH with ![pH=-log[H^{+}]=-log(6.09x10^{-4} )=3.2](/tpl/images/0224/4748/e6968.png)