
Chemistry, 06.09.2019 18:10 itsyagirl11076
For the overall reaction below, which of the following is the correctly written rate law? overall reaction: o3(g)+2no2(g)→n2o5(g)+o2(g) step 1: o3(g)+no2(g)→no3(g)+o2(g) slow step 2: no3(g)+no2(g)→n2o5(g) fast view available hint(s) for the overall reaction below, which of the following is the correctly written rate law? overall reaction: step 1: slow step 2: fast rate=k[o3][no2]2 rate=k[no3][no2] rate=k[o3][no2] rate=k[o3][no2]2[n2o5][o2]

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1
You know the right answer?
For the overall reaction below, which of the following is the correctly written rate law? overall r...
Questions

Mathematics, 14.01.2021 17:00


Mathematics, 14.01.2021 17:00





History, 14.01.2021 17:00

Arts, 14.01.2021 17:00

History, 14.01.2021 17:00


English, 14.01.2021 17:00


Computers and Technology, 14.01.2021 17:00



Computers and Technology, 14.01.2021 17:00



Computers and Technology, 14.01.2021 17:00

![Rate=k[O_3][NO_2]^2](/tpl/images/0224/5146/9d0a3.png)
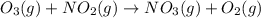 slow
slow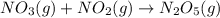 fast
fast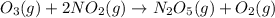
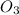 is 1 and Order with respect to
is 1 and Order with respect to 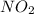 is 2.
is 2.

