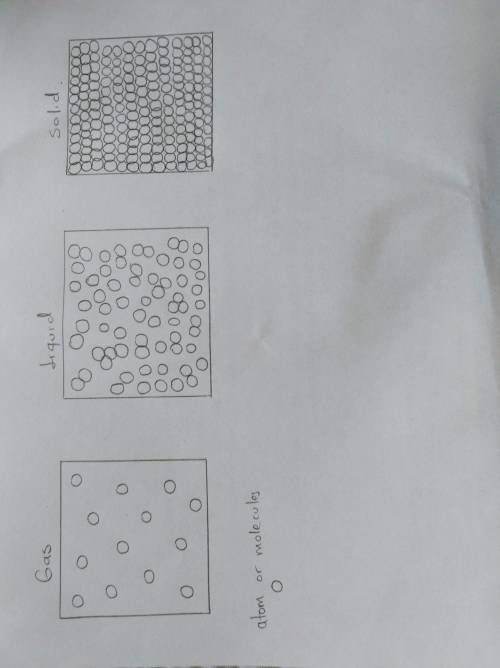
What are the three states of matter 2. draw the different states of matter at the molecular level (pick a symbol to represent an atom or draw on whiteboard and insert a picture of each) solid liquid gas 3. describe the mass, shape, volume and compressibility of a gas. 4. what happens to a gas as it is heated? 5. describe the mass, shape, volume and compressibility of a liquid. 6. what happens to a liquid as it is heated? 7. describe the mass, shape, volume and compressibility of a solid. 8. what happens to a solid as it is heated?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identifying limitations of kinetic-molecular theorya chemist is studying the properties of a gas under various conditions. he observes that when the gas is at room temperature and low pressure, it behaves as an ideal gas. when the gas is cooled to 10 kelvin and is placed under high pressure, however, it deviates significantly from an ideal .
Answers: 1

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
What are the three states of matter 2. draw the different states of matter at the molecular level (p...
Questions


Mathematics, 17.12.2020 02:50

Mathematics, 17.12.2020 02:50





Mathematics, 17.12.2020 02:50


Social Studies, 17.12.2020 02:50

Arts, 17.12.2020 02:50

Mathematics, 17.12.2020 02:50


Mathematics, 17.12.2020 02:50


Mathematics, 17.12.2020 02:50

Mathematics, 17.12.2020 02:50


Business, 17.12.2020 02:50