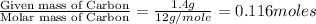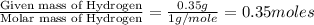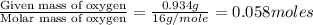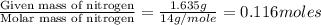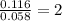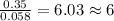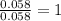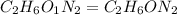Dimethyl nitrosamine is a known carcinogen. it can be formed in the intestinal tract when digestive juices react with the nitrite ion is preserved and smoked meats. it is made up of carbon, hydrogen, nitrogen, and oxygen atoms. a 4.319 g sample of dimethyl nitrosamine burned in oxygen yields 5.134 g of co2 and 3.173 g of h2 o. the compound contains 37,82% by mass of nitrogen. what is the empirical formula of dimethyl nitrosamine?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1


Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2
You know the right answer?
Dimethyl nitrosamine is a known carcinogen. it can be formed in the intestinal tract when digestive...
Questions

Physics, 05.05.2020 02:46



Biology, 05.05.2020 02:46

Mathematics, 05.05.2020 02:46

Geography, 05.05.2020 02:46


Mathematics, 05.05.2020 02:46

Mathematics, 05.05.2020 02:46


Mathematics, 05.05.2020 02:46

Spanish, 05.05.2020 02:46

Mathematics, 05.05.2020 02:46

Mathematics, 05.05.2020 02:46






Chemistry, 05.05.2020 02:46

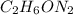
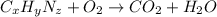
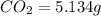
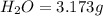
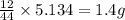 of carbon will be contained.
of carbon will be contained.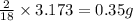 of hydrogen will be contained.
of hydrogen will be contained.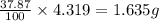 of nitrogen
of nitrogen