
Chemistry, 06.09.2019 20:30 silvajimena74
Enter your answer in the provided box. the atomic masses of 63cu (69.17 percent) and 65cu (30.83 percent) are 62.94 and 64.93 amu, respectively. calculate the average atomic mass of copper. the percentages in parentheses denote the relative abundances.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
Enter your answer in the provided box. the atomic masses of 63cu (69.17 percent) and 65cu (30.83 per...
Questions




History, 06.04.2021 17:50


Biology, 06.04.2021 17:50

Computers and Technology, 06.04.2021 17:50

Spanish, 06.04.2021 17:50

Mathematics, 06.04.2021 17:50

Mathematics, 06.04.2021 17:50


World Languages, 06.04.2021 17:50

History, 06.04.2021 17:50

Chemistry, 06.04.2021 17:50



History, 06.04.2021 17:50

Spanish, 06.04.2021 17:50


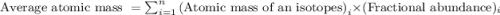 .....(1)
.....(1)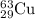 isotope:
isotope: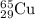 isotope:
isotope:![\text{Average atomic mass of Copper}=[(62.94\times 0.6917)+(64.93\times 0.3083)]\\\\\text{Average atomic mass of copper}=63.55amu](/tpl/images/0224/6800/fff18.png)



