

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3
You know the right answer?
Consider the reaction of cacn2 and water to produce caco3 and nh3: cacn2 + 3 h2o → caco3 + 2 nh3 ....
Questions

Mathematics, 20.10.2019 11:10

Mathematics, 20.10.2019 11:10

Mathematics, 20.10.2019 11:10



History, 20.10.2019 11:10






Social Studies, 20.10.2019 11:10






Mathematics, 20.10.2019 11:10

Mathematics, 20.10.2019 11:10

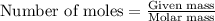
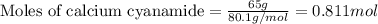
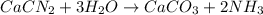
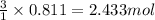 of water.
of water.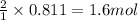 of ammonia.
of ammonia.

