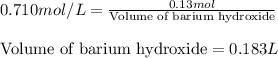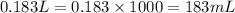Chemistry, 06.09.2019 22:10 zmoore8015
Calculate the number of milliliters of 0.710 m ba(oh)2 required to precipitate all of the mn2+ ions in 161 ml of 0.796 m kmno4 solution as mn(oh)2. the equation for the reaction is: mnso4(aq) + ba(oh)2(aq) mn(oh)2(s) + baso4(aq)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2
You know the right answer?
Calculate the number of milliliters of 0.710 m ba(oh)2 required to precipitate all of the mn2+ ions...
Questions














Chemistry, 30.07.2019 19:30

History, 30.07.2019 19:30

Chemistry, 30.07.2019 19:30




History, 30.07.2019 19:30

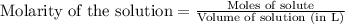 .....(1)
.....(1)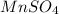 = 0.796 M
= 0.796 M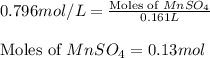
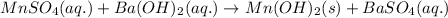
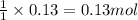 of barium hydroxide
of barium hydroxide