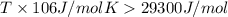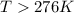Chemistry, 06.09.2019 22:30 muanghoih14
For a reaction for which ah = +29.3 kj/mol and as = +106 j/mol•k, which of the following statements is true? a) the reaction is spontaneous above 276 k. b) the reaction is spontaneous below 276 k. c) the reaction will never reach equilibrium. d) the reaction will never be spontaneous.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
For a reaction for which ah = +29.3 kj/mol and as = +106 j/mol•k, which of the following statements...
Questions


Health, 17.03.2021 23:50

Geography, 17.03.2021 23:50


Arts, 17.03.2021 23:50

History, 17.03.2021 23:50





Chemistry, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50

English, 17.03.2021 23:50


Mathematics, 17.03.2021 23:50




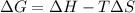
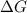 = Gibbs free energy
= Gibbs free energy 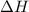 = enthalpy change = +29.3 kJ/mol =29300 J/mol
= enthalpy change = +29.3 kJ/mol =29300 J/mol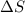 = entropy change = +106 J/molK
= entropy change = +106 J/molK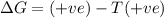
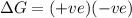
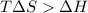 for reaction to be spontaneous
for reaction to be spontaneous