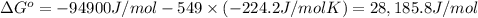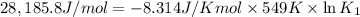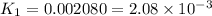Chemistry, 06.09.2019 23:20 zhellyyyyy
Determine the equilibrium constant for the following reaction at 549 k. ch2o(g) + 2 h2(g) → ch4(g) + h2o(g) δh° = -94.9 kj; δs°= -224.2 j/k determine the equilibrium constant for the following reaction at 549 k. ch2o(g) + 2 h2(g) → ch4(g) + h2o(g) δh° = -94.9 kj; δs°= -224.2 j/k 1.07 x 109 481 2.08 x 10-3 9.35 x 10-10 1.94 x 10-12

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2


Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
Determine the equilibrium constant for the following reaction at 549 k. ch2o(g) + 2 h2(g) → ch4(g) +...
Questions

Social Studies, 13.12.2020 15:00

Chemistry, 13.12.2020 15:00


Social Studies, 13.12.2020 15:00


Mathematics, 13.12.2020 15:00


Mathematics, 13.12.2020 15:00

Biology, 13.12.2020 15:00




Mathematics, 13.12.2020 15:00


Mathematics, 13.12.2020 15:00

Mathematics, 13.12.2020 15:00

Business, 13.12.2020 15:00


Mathematics, 13.12.2020 15:00

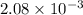 .
.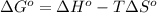
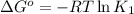
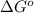 = Gibbs free energy
= Gibbs free energy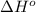 = Enthalpy of reaction
= Enthalpy of reaction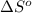 = Entropy of reaction
= Entropy of reaction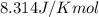
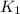 = equilibrium constant at T
= equilibrium constant at T