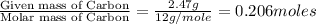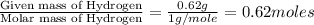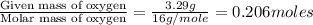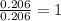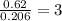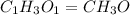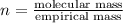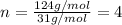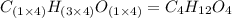Chemistry, 06.09.2019 23:20 zoewilliamss26
Ethylene glycol used in automobile antifreeze and in the production of polyester. the name glycol stems from the sweet taste of this poisonous compound. combustion of 6.38 g of this compound gives 9.06 g of co2 and 5.58 g of h2o. the compound only contains c, h, and o. if the molecular mass of this compound is 124 amu, what is the empirical and molecular formula?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
You know the right answer?
Ethylene glycol used in automobile antifreeze and in the production of polyester. the name glycol st...
Questions

History, 24.08.2019 11:30

Biology, 24.08.2019 11:30



Mathematics, 24.08.2019 11:30

Social Studies, 24.08.2019 11:30


Mathematics, 24.08.2019 11:30


Chemistry, 24.08.2019 11:30

History, 24.08.2019 11:30


Computers and Technology, 24.08.2019 11:30

Biology, 24.08.2019 11:30



SAT, 24.08.2019 11:30


History, 24.08.2019 11:30

Mathematics, 24.08.2019 11:30

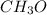 and
and 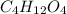
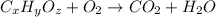
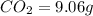
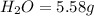
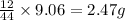 of carbon will be contained.
of carbon will be contained.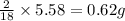 of hydrogen will be contained.
of hydrogen will be contained.