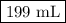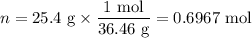Chemistry, 06.09.2019 23:30 kloekamakeeaina14
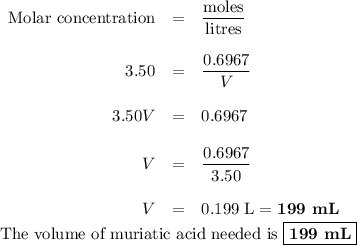
Muriatic acid, an industrial grade of concentrated hcl, is used to clean masonry and cement. its concentration is 11.7 m. for routine use, a diluted solution of 3.50 m is prepared. how many milliliters of 3.50 m muriatic acid solution contain 25.4 g of hcl?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 09:00
Chortling is used to clean water. another possible atom that would also work is a. sodium b. sulfur c. bromine
Answers: 1
You know the right answer?
Muriatic acid, an industrial grade of concentrated hcl, is used to clean masonry and cement. its con...
Questions



Arts, 25.04.2020 22:17

Health, 25.04.2020 22:17

Mathematics, 25.04.2020 22:17

Biology, 25.04.2020 22:17


Mathematics, 25.04.2020 22:17

Mathematics, 25.04.2020 22:17





Mathematics, 25.04.2020 22:17



Mathematics, 25.04.2020 22:18

Computers and Technology, 25.04.2020 22:18


World Languages, 25.04.2020 22:18