
Chemistry, 07.09.2019 01:10 jiedwards3835
For the reaction pc15 (8) pc13 (g) + cl2 (g) k = 0.0454 at 261 °c. if a vessel is filled with these gases such that the initial concentrations are [pc15) = 0.20 m, [pc13] = 0.20 m, and (cl21 = 2.5 m, in which direction will a reaction occur and why? a) toward products because qc = 0.56 b) toward reactants because qc = 2.5 c) toward products because qc = 2.8 d) toward reactants because qc = 0.0454 e) it is at equilibrium because qc = 1

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1


Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
For the reaction pc15 (8) pc13 (g) + cl2 (g) k = 0.0454 at 261 °c. if a vessel is filled with these...
Questions

English, 09.12.2020 14:00



History, 09.12.2020 14:00


Mathematics, 09.12.2020 14:00

History, 09.12.2020 14:00


French, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00


Business, 09.12.2020 14:00


Mathematics, 09.12.2020 14:00

Physics, 09.12.2020 14:00

Business, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00


Physics, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00

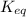 is samller than
is samller than 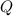 of the reaction . So,the reaction will shift towards the left i.e. towards the reactant side.
of the reaction . So,the reaction will shift towards the left i.e. towards the reactant side.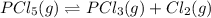
![Q=\frac{[PCl_3][Cl_2]}{[[PCl_5]^1}](/tpl/images/0224/9376/2eff0.png)
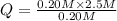
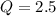
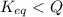 , the reaction will shift towards the left i.e. towards the reactant side.
, the reaction will shift towards the left i.e. towards the reactant side.

