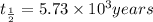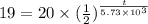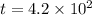Chemistry, 07.09.2019 02:30 castiaulii16
The half life for the decay of carbon-14 is 5.73 x 10 years. suppose the activity due to the radioactive decay of the carbon-14 in a tiny sample of an artifact made of wood from an archeological dig is measured to be 19. bq. the activity in a similar-sized sample of fresh wood is measured to be 20. bq. calculate the age of the artifact. round your answer to 2 significant digits. years x 5 ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

You know the right answer?
The half life for the decay of carbon-14 is 5.73 x 10 years. suppose the activity due to the radioac...
Questions



Social Studies, 24.10.2021 09:00


History, 24.10.2021 09:00

English, 24.10.2021 09:00


Mathematics, 24.10.2021 09:00

Biology, 24.10.2021 09:00

Mathematics, 24.10.2021 09:00


Computers and Technology, 24.10.2021 09:00

Geography, 24.10.2021 09:00

History, 24.10.2021 09:00



Mathematics, 24.10.2021 09:10

Mathematics, 24.10.2021 09:10


 years
years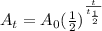
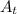 represents activity of radioactive nuclide after t time,
represents activity of radioactive nuclide after t time, 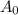 represents initial activity of radioactive nuclide and
represents initial activity of radioactive nuclide and 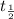 represents half-lifeHere,
represents half-lifeHere, 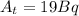 ,
, 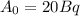 and
and