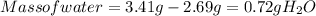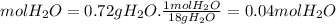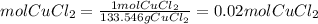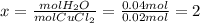Chemistry, 07.09.2019 04:30 batmannn1516
Ahydrate of copper (ii) chloride has the following formula: cucl2 - x h2o. the water in a 3.41-g sample of the hydrate was driven off by heating. the remaining sample had a mass of 2.69 g . find the number of waters of hydration (x) in the hydrate.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:40
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1

Chemistry, 23.06.2019 08:00
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1
You know the right answer?
Ahydrate of copper (ii) chloride has the following formula: cucl2 - x h2o. the water in a 3.41-g sa...
Questions

Computers and Technology, 17.07.2019 07:30

Social Studies, 17.07.2019 07:30

Computers and Technology, 17.07.2019 07:30

History, 17.07.2019 07:30





Social Studies, 17.07.2019 07:30

Mathematics, 17.07.2019 07:30

Geography, 17.07.2019 07:30

History, 17.07.2019 07:30


Biology, 17.07.2019 07:30

Health, 17.07.2019 07:30


Social Studies, 17.07.2019 07:30

Social Studies, 17.07.2019 07:30


Business, 17.07.2019 07:30

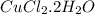
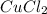 is the mass of remaining sample, because it is a product of loss of drying from initial sample. This means that the mass of water is the mass has been lost.
is the mass of remaining sample, because it is a product of loss of drying from initial sample. This means that the mass of water is the mass has been lost.