Chemistry, 07.09.2019 05:21 Lilleypad07
A0.418 g sample of kcl is added to 35.2 g of water in a calorimeter. if the temperature decreases by 1.01°c, what is the approximate amount of heat (in j) involved in the dissolution of the kcl, assuming the heat capacity of the resulting solution is 4.18 j/g°c? j is the reaction exothermic or endothermic?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
A0.418 g sample of kcl is added to 35.2 g of water in a calorimeter. if the temperature decreases by...
Questions

Mathematics, 03.09.2020 04:01


History, 03.09.2020 04:01


Mathematics, 03.09.2020 04:01


Mathematics, 03.09.2020 04:01


Engineering, 03.09.2020 04:01





History, 03.09.2020 04:01

Social Studies, 03.09.2020 04:01


English, 03.09.2020 04:01

History, 03.09.2020 04:01


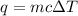
 = 1.01 °C
= 1.01 °C