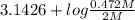Chemistry, 07.09.2019 05:30 brookebeatrice8
Calculate the ph of a solution that is 2.00 m hf, 1.00 m naoh, and 0.472 m naf. (ka=7,2×104)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Calculate the ph of a solution that is 2.00 m hf, 1.00 m naoh, and 0.472 m naf. (ka=7,2×104)...
Questions

History, 02.10.2020 23:01

Mathematics, 02.10.2020 23:01

Mathematics, 02.10.2020 23:01




Mathematics, 02.10.2020 23:01

Chemistry, 02.10.2020 23:01

History, 02.10.2020 23:01

Mathematics, 02.10.2020 23:01

Medicine, 02.10.2020 23:01


Mathematics, 02.10.2020 23:01

Mathematics, 02.10.2020 23:01

Advanced Placement (AP), 02.10.2020 23:01




Mathematics, 02.10.2020 23:01

English, 02.10.2020 23:01

 =
= 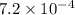
![pK_{a} + log\frac{[salt]}{[acid]}](/tpl/images/0225/1364/8b24d.png)
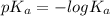
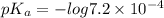
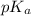 = 3.1426
= 3.1426